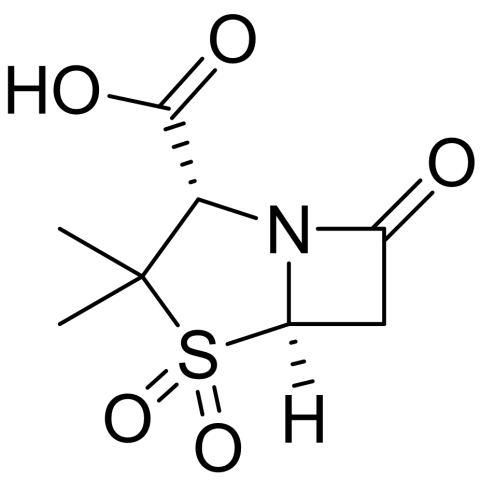
Sulbactam is a β-lactamase inhibitor developed in the late 1970s to counter bacterial resistance caused by β-lactamase enzymes. Although it has little antibacterial activity alone, it protects β-lactam antibiotics like ampicillin and cefoperazone from enzymatic breakdown, restoring their effectiveness against resistant bacteria. Introduced in the early 1980s as combinations such as ampicillin/sulbactam (Unasyn) and cefoperazone/sulbactam (Sulperazon), it remains widely used for treating respiratory, urinary, intra-abdominal, and skin infections caused by β-lactamase–producing organisms.
BRAND NAMES
Some brand names for sulbactam include Unasyn (amipicillin/sulbactam), Xacduro (durlobactam/sulbactam), and various combinations with other antibiotics, such as Sulbamax, Sulbacip, and Marzon SB.
Sulbactam with ampicillin:
Unasyn, Sulbamax, Sulbacip, Sulbamax, Addbactam, and Marzon SB.
Sulbactam with other antibiotics:
Xacduro: (durlobactam/sulbactam)
Sulperazon: (cefoperazone/sulbactam)
Zostum: (cefoperazone/sulbactam)
Swapenem-SB: (meropenem/sulbactam)
C-XON SB: (ceftriaxone/sulbactam)
Uniceft-S: (ceftriaxone/sulbactam)
Arixone-S: (ceftriaxone/sulbactam)
MECHANISM OF ACTION
Sulbactam is a β-lactamase inhibitor that protects β-lactam antibiotics like ampicillin from being inactivated by bacterial enzymes. It does this by irreversibly binding to and blocking β-lactamase enzymes, which would otherwise degrade the antibiotic. This inhibition allows the companion antibiotic to retain its ability to disrupt bacterial cell wall synthesis and effectively eliminate the bacteria.
PHARMACOKINETICS
Absorption
Sulbactam is mainly administered by intravenous (IV) or intramuscular (IM) routes because it is poorly absorbed when taken orally. After IV infusion, peak plasma concentrations occur rapidly, while IM injection provides high bioavailability with peak levels reached within 1 to 2 hours, similar to ampicillin. For oral use, sulbactam is given as the prodrug sultamicillin, which is hydrolyzed during absorption to release both sulbactam and ampicillin into systemic circulation.
Distribution
Sulbactam distributes widely into extracellular fluids and tissues, exhibiting a steady-state volume of distribution of approximately 12.2–16.3 L.
Metabolism
Sulbactam is minimally metabolized, with roughly 75% of a parenteral dose excreted unchanged in the urine. The remainder undergoes limited metabolism, though the pathways are not well defined. Its elimination is mainly renal, through glomerular filtration and tubular secretion, and it has an approximate half-life of one hour in healthy adults.
Excretion
Sulbactam is primarily eliminated unchanged in the urine, with about 75% of a parenteral dose excreted renally. The elimination occurs via glomerular filtration and tubular secretion. Only a small fraction undergoes metabolism. In healthy adults, the half-life of sulbactam is approximately 1 hour.
PHARMACODYNAMICS
Sulbactam is a β-lactamase inhibitor that has minimal antibacterial activity by itself, except for some intrinsic activity against Acinetobacter baumannii. Its main pharmacodynamic role is to protect co-administered β-lactam antibiotics from degradation by bacterial β-lactamase enzymes.
ADMINISTRATION
Sulbactam is primarily administered parenterally due to poor oral absorption. The common routes are:
Intravenous (IV) injection or infusion: Provides rapid and complete systemic absorption, often used in hospitals for serious infections.
Intramuscular (IM) injection: Offers high bioavailability with peak plasma levels reached in about 1–2 hours, similar to ampicillin.
For oral use, sulbactam is given as the prodrug sultamicillin, which is absorbed and hydrolyzed in the body to release both sulbactam and ampicillin.
DOSAGE AND STRENGTH
Sulbactam is mainly used in combination with β-lactam antibiotics such as ampicillin or cefoperazone.
Ampicillin + Sulbactam (Unasyn): Common injection strengths are 1.5 g (1 g ampicillin + 0.5 g sulbactam) and 3 g (2 g ampicillin + 1 g sulbactam) per dose.
Cefoperazone + Sulbactam (Sulperazon): Typically given in a 1:1 ratio, e.g., 1 g + 1 g or 2 g + 2 g per dose.
Adult dosing: Usually administered every 6–12 hours, adjusted according to renal function and infection severity.
Pediatric dosing: Weight-based, approximately 25–50 mg/kg/day of sulbactam, divided into multiple doses.
Oral administration (as sultamicillin): Adult doses range from 375 mg to 750 mg per dose, given every 12 hours, providing both ampicillin and sulbactam.
FOOD INTERACTIONS
Sulbactam has minimal direct food interactions, especially when administered parenterally via intravenous or intramuscular routes. When given orally as the prodrug sultamicillin, food may slightly affect its absorption, but this rarely has a clinically significant impact. High-fat meals might delay absorption of the oral formulation, yet overall efficacy is generally maintained. Therefore, oral sulbactam can be taken with or without food, while food does not affect its effectiveness when given by injection.
DRUG INTERACTIONS
Sulbactam may interact with several drugs, including antibiotics like gentamicin, as well as probenecid and methotrexate. These interactions can increase toxicity or modify its effectiveness by influencing its excretion. In some cases, dose adjustments or careful timing of administration may be necessary to avoid adverse effects.
CONTRAINDICATIONS
Sulbactam is contraindicated in patients with a known hypersensitivity to sulbactam, penicillins, or other β-lactam antibiotics. It should be used with caution or avoided in individuals with severe renal impairment unless doses are properly adjusted. Patients with a history of serious allergic reactions, such as anaphylaxis to β-lactam antibiotics, should not receive sulbactam. Certain formulations may also be unsuitable for neonates or premature infants, requiring careful dosing. Additionally, combining sulbactam with drugs that have known adverse interactions should be avoided unless under strict medical supervision.
SIDE EFFECTS
Common side effects:
Nausea, vomiting, and diarrhea.
Headache and fatigue.
Injection site pain, swelling, or redness.
Rash.
Serious side effects:
Severe allergic reaction.
Liver problems.
Kidney problems.
Severe diarrhea.
OVERDOSE
Nausea.
Vomiting.
Diarrhea.
Abdominal discomfort.
Dizziness.
Headache.
Lethargy
Seizures.
TOXICITY
Sulbactam is a beta-lactamase inhibitor with low toxicity, but it can cause adverse effects, particularly at high doses, in patients with pre-existing conditions, or as part of an allergic reaction. It is almost always administered in combination with another antibiotic, such as ampicillin or cefoperazone, and many side effects are associated with the combination drug rather than sulbactam alone.