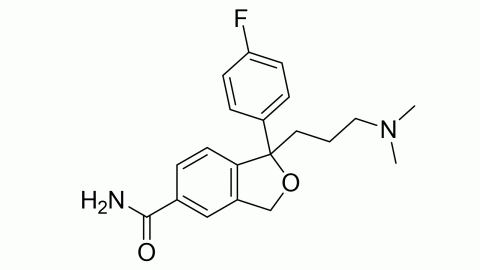
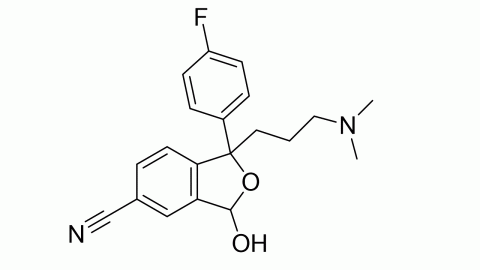
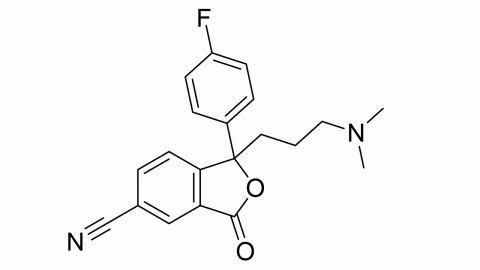
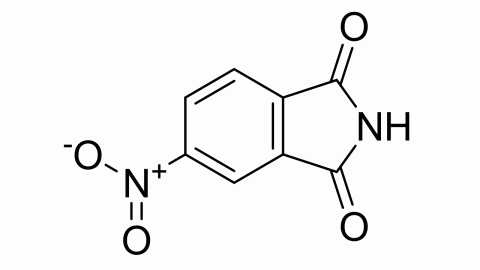
Filter sub products categories alphabetically
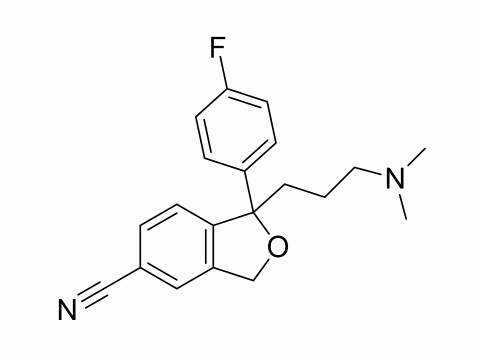
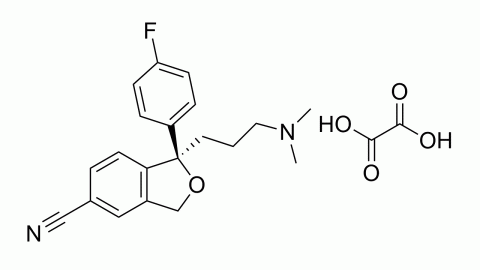
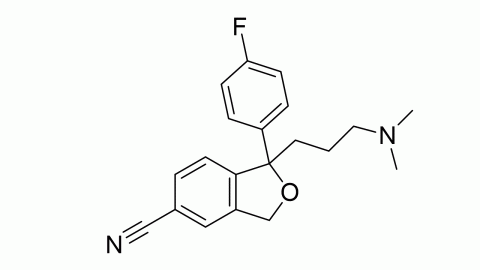
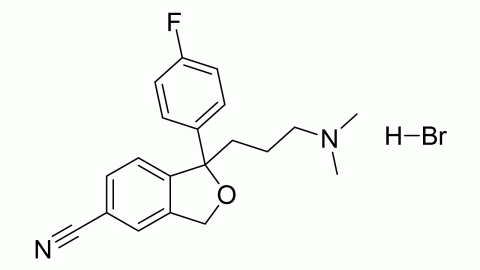
Citalopram is an antidepressant that acts as a selective serotonin reuptake inhibitor. It is most typically used to treat depression, but it can also be used to treat anxiety, obsessive-compulsive disorder, and panic attacks. It helps many people recover from depression while having fewer side effects than earlier antidepressants. The Food and Drug Administration of the United States has approved it for the treatment of severe depressive disorders.
BRAND NAMES:
Lexapro is in oral dosage form as a tablet. This is available in strengths of 5 mg, 10 mg, and 20 mg.
It is also available in the form of oral solution, available in the strength of 5 mg/ml.
MECHANISM OF ACTION:
Citalopram is the active enantiomer of citalopram, a racemic medication. In vitro and in vivo investigations have shown that citalopram is a highly powerful and selective serotonin reuptake inhibitor that works by specifically inhibiting the serotonin membrane transporter. In addition to its high affinity for the principal bonding site, citalopram has a 1000-fold reduced affinity for a secondary binding site on the serotonin transporter. Citalopram has little-to-no binding affinity for a number of additional receptors, including histamine and muscarinic receptors, and minimal action at these off-targets may account for some of its side effects.
PHARMACOKINETICS:
Absorption: Peak plasma levels of citalopram occur around 4 hours after an oral dose is administered to healthy individuals. Citalopram is expected to be absorbed virtually completely following oral administration and is unaffected by meals.
Distribution: The estimated amount of distribution is basically 12–26 L/kg.
Metabolism: Citalopram is converted in the liver into S-desmethylcitalopram and s-di-de methylcitalopram. In humans, unaltered citalopram is the most abundant component in plasma. After numerous doses of citalopram, the average plasma concentrations of the metabolites S-DCT and S-DDCT are typically 28–31% and 5% of the parent drug concentration, respectively. In vitro investigations indicate that metabolites play no important role in citalopram's therapeutic activities.
Excretion: Following oral administration of citalopram, approximately 8% of the entire dose is removed in the urine as unaltered citalopram.
PHARMACODYNAMICS:
Citalopram is an example of the group of drugs known as selective serotonin reuptake inhibitors. By inhibiting serotonin's reuptake into the presynaptic terminal of serotonergic neurons, these substances raise the amount of serotonin in neuronal synapses. Because of its strength, it seems to have a rather early beginning of effect when compared to other SSRIs. Patients receiving concurrent therapy with other serotonergic medications or those with a higher-than-baseline risk of bleeding should use citalopram with caution. If citalopram is abruptly stopped, it can potentially result in a discontinuation syndrome. If stopping therapy is necessary, it should be tapered gradually.
ADMINISTRATION:
Escitalopram is typically taken once a day, with or without food. The standard first dose of the medicine is 10 mg, with the possibility of increasing after 1 week for symptom relief. When switching from another SSRI to citalopram, it is recommended to reduce the dose for four weeks.
DOSAGE AND STRENGTHS:
Citalopram is administered orally and is available in 2 forms: an oral solution at a concentration of 1 mg/mL and tablets in 5 mg, 10 mg, and 20 mg strengths.
CONTRAINDICATIONS:
Before prescribing citalopram, a complete risk assessment should be performed, taking into account hypersensitive reactions to other drugs, particularly antidepressants and SSRIs, potential QT prolongation, and the risk of serotonin syndrome in all individuals. Notably, this medicine is not recommended for patients who have had previous hypersensitivity responses to.
DRUG INTERACTIONS:
Citalopram oral tablet may interact with other medications, supplements, or herbs that you are taking. An interaction happens when a substance influences how a drug works. This can be dangerous or stop the medication from working correctly.
Blood thinners—Warfarin
Non-steroidal anti-inflammatory:
Diclofenac
Etodolac
Ibuprofen
Indomethacin
Ketorolac
Naproxen
Migraine drugs:
Almotriptan
Eletriptan
rizatriptan
Psychiatric drugs:
isocarboxazid
Phenelzine
Anti-depressant drugs:
Citalopram
Fluoxetine
Paroxetine
Sertraline
Diuretics:
Furosemide
Hydrochlorothiazide
Spironolactone
SIDE EFFECTS:
- Nausea
- Diarrhea
- Constipation
- Sexual problems in males and females
- Drowsiness
- Yawning
- Shaking
- Dizziness
- Increased sweating
- Dry mouth
- Heart burn
- Runny nose
- Sneezing
OVERDOSE:
There is no particular therapy for citalopram toxicity. The symptoms of overdosing include:
Symptoms include:
- vomiting and dizziness
- Seizures
- Coma
- Nausea
TOXICITY:
Since citalopram is well dispersed into tissue after oral administration, it is unlikely to be helpful to extract the medication from plasma using forced diuresis, dialysis, or other means. A specific counteradote for citalopram overdose does not exist. Monitoring for cardiac irregularities, changing vital signs, and administering treatment with supportive measures when necessary should be the main goals of overdose management.
STORAGE CONDITIONS:
Store at 20-25°C (68°-77°F). excursions permitted to 15-30⁰C (59-86⁰F).